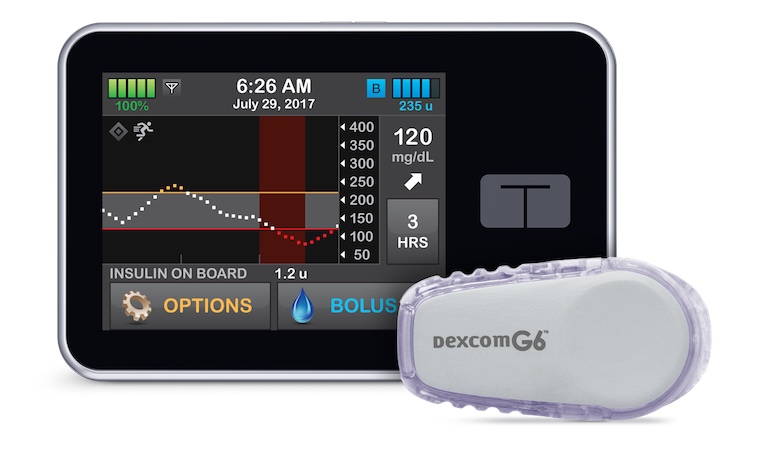
The Control-IQ artificial pancreas system was derived from research done at the Center for Diabetes Technology at the University of Virginia. Image credit: Tandem Diabetes Care
An artificial pancreas originally developed at the University of Virginia Center for Diabetes Technology improves blood sugar control in children ages 2 to 6 with type 1 diabetes, according to a new study. Details of the clinical study and its findings were just published in the prestigious New England Journal of Medicine.
Trial participants using the artificial pancreas spent approximately three more hours per day in their target blood sugar range compared with participants in a control group who continued relying on the methods they were already using to manage their blood sugar.
The Control-IQ system, manufactured by Tandem Diabetes Care, is a diabetes management device that automatically monitors and regulates blood glucose. The artificial pancreas has an insulin pump that uses advanced control algorithms based on the person’s glucose-monitoring information to adjust the insulin dose as needed.
Based on findings from two earlier studies, the system has previously been approved by the U.S. Food and Drug Administration for people ages 6 and older with type 1 diabetes.
“After the resounding success of Control-IQ technology in people ages 6 and up, it is very rewarding to see our youngest patients, and often the most challenging patients to help, benefit as well,” said Marc D. Breton, PhD, a UVA School of Medicine researcher who served as the trial’s principal investigator and was recently honored as UVA’s 2022 Innovator of the Year. “With these results, we have now accumulated years of clinical validation of this system across all age groups and look forward to seeing this life-changing technology made available to the broadest possible population.”
Used During Everyday Life
The study enrolled 102 children between ages 2 and 6 at three U.S. sites (UVA, Stanford University and the University of Colorado) and randomly assigned 68 of them to use the artificial pancreas system for 13 weeks, while the remaining 34 children were assigned to the control group. All participants maintained their regular daily routines during the study.
On average, the time participants using the artificial pancreas spent within their target blood glucose range was about 12 percentage points higher than participants in the control group overall and 18 percentage points higher during the overnight hours of 10 p.m. to 6 a.m. Nighttime blood glucose control is particularly important, as severe, unchecked hypoglycemia (very low blood glucose levels) can lead to seizures, coma or even death.
Overall, the researchers found, participants were able to use the artificial pancreas safely. There were two cases of severe hypoglycemia in the artificial pancreas group, compared with one in the control group. There was also one case of diabetic ketoacidosis in the artificial pancreas group, caused by a failure of the thin plastic tube that connects the insulin pump to the patient’s body.
Of note, most of the study-related visits – including 80% of the training sessions on the artificial pancreas and more than 90% of the overall visits – were conducted virtually. Achieving the reported results under these conditions highlights the ease of use of the technology and its potential for areas without easy access to endocrinologists.
“At the end of the day, this technology significantly improved glycemia and ensured safety of our youngest patients, but perhaps just as importantly it lessened these families’ constant anxiety about glucose levels, especially during the night.” Breton said. “It is incredibly rewarding for us to hear about these families’ experiences and how they manage to integrate these new tools in their life, offering some reprieve to the challenges they face.”
Findings Published
The study results have been published in the New England Journal of Medicine. The study’s authors are R. Paul Wadwa, Zachariah W. Reed, Bruce A. Buckingham, Mark D. DeBoer, Laya Ekhlaspour, Gregory P. Forlenza, Melissa Schoelwer, John Lum, Craig Kollman, Roy W. Beck and Breton.
This study was funded by the National Institutes of Health’s National Institute of Diabetes and Digestive and Kidney Diseases, grant U01DK127551. Tandem Diabetes Care provided the investigational closed-loop systems used in the trial, while Dexcom, Inc. provided the continuous glucose monitor supplies used in the trial.