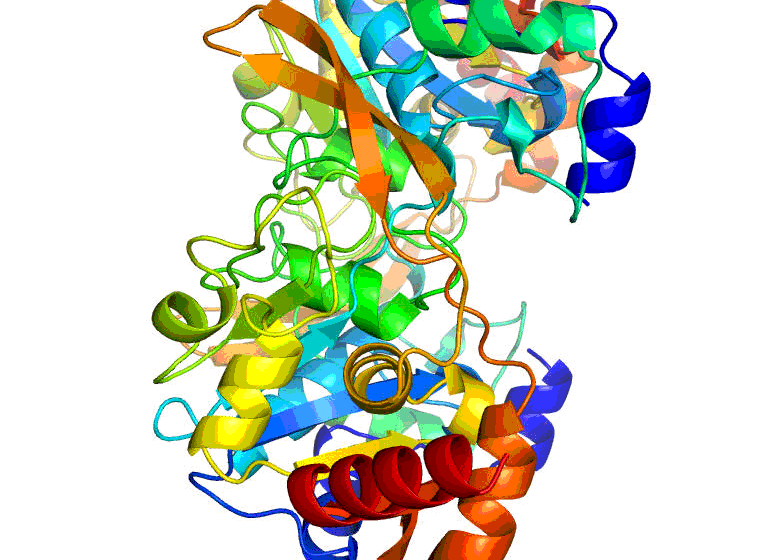
Researchers at the University of Virginia Health System have attained a major milestone in their work for the Center for Structural Genomics of Infectious Diseases (CSGID). Last week, they mapped out a three-dimensional structure of the enzyme protein BA2930, which is produced by the bacteria responsible for anthrax, Bacillus anthracis . They did so by using X-ray crystallography, a technique that provides a three-dimensional snapshot of the arrangement of atoms in a protein.
“Having a map of the BA2930 protein structure, in particular, can help us understand the mechanism by which deadly bacteria like anthrax develop resistance to the antibiotics used to combat them,” explains Wladek Minor, PhD, leader of the UVA team and professor of Molecular Physiology and Biological Physics. BA2930 is believed to play a major role in anthrax’s resistance mechanism to the aminoglycoside family of antibiotics, including streptomycin, gentamycin, and kanamycin. Minor believes that insights gained from the study of BA2930 are likely to apply to other disease-causing bacteria with similar protein structures.
The newly-visualized structure is bound with a naturally-found biological molecule known as coenzyme A, which is required for enzyme protein functioning. Previously, the UVA group mapped out the structure of the BA2930 protein by itself.
BA2930 is an important milestone in the work of the CSGID because it is the 100 th three-dimensional structure deposited in its Protein Data Bank, or PDB. The PDB is accessible to scientists and developers of drugs, vaccines and medical diagnostics around the world.
Minor’s group works only on the individual proteins of disease-causing bacteria such as Bacillus anthracis , which by themselves are not virulent. Whole bacteria are needed to trigger the infectious disease.
The team is now mapping the structure of the protein bound to aminoglycoside molecules and has already produced some computer models showing how those interactions occur. Performed in collaboration with Professor Alexei Savchenko of the University of Toronto, this new research is expected to advance understanding of how the mechanisms of resistance to aminoglycoside antibiotics function.
Established in 2007, CSGID is one of two centers funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to determine the structures of proteins from major human pathogens. A key CSGID goal is to enhance understanding of the molecular mechanisms by which salmonella, staph infections, plague, cholera, anthrax and other dreaded diseases may interact with potential new treatments.
Thanks to improved techniques, including HKL-3000 software developed by Minor’s group in collaboration with Prof. Otwinowski from UT Southwestern, the pace of the CSGID’s work is accelerating. Between 2010 and 2012, the consortium expects to add about 100 protein structures to the PDB each year.
Minor says, “The efforts of the CSGID as a whole are providing crucial molecular structure information about proteins in a wide variety of dangerous pathogens, which in the future can be used to develop new antibiotics and other drugs for the treatment of the diseases they cause.”
CSGID is led by Professor Wayne Anderson at Northwestern University. In addition to Northwestern, UVA and the University of Toronto, CSGID collaborators include the J. Craig Venter Institute, University of Chicago, University of Texas Southwestern Medical Center, Washington University and University College London.
CSGID is funded with Federal funds from the National Institute of Allergy and Infectious Diseases under Contract No. HHSN272200700058C.


